Bildnachweis: CorTec.
Freiburg-based CorTec GmbH has achieved a significant milestone in neurotechnology: this summer, the company’s Brain Interchange™ system was implanted in a patient in the United States – the first in human implantation of the system. The individual, a stroke survivor who remained severely impaired despite conventional rehabilitation, is now part of a clinical study that aims to enhance motor recovery through targeted brain stimulation combined with occupational therapy.
Our system records brain signals and delivers precise electrical impulses, much like a conversation; the device responds to brain activity in real time,’ explains CEO Dr Frank Desiere. This implantation marks the beginning of a new era: for the first time, a fully German-developed BCI system is being evaluated in a US clinical trial, a milestone not only for CorTec, but for European neurotechnology as a whole.
Closed-Loop instead of static stimulation

At the heart of CorTec’s innovation lies a closed-loop brain-computer interface. Unlike systems that stimulate on fixed parameters, CorTec’s approach continuously processes neural signals and adjusts stimulation dynamically. While competitors such as Neuralink rely on needle-like electrodes penetrating brain tissue, CorTec uses surface electrodes, which are less invasive and designed to avoid tissue damage. ‘Many competitors rely on rigid, one-sizefits-all stimulation. We are opening up a new field of personalised neurotherapy that adapts precisely to each patient,’ says Desiere.
Broad spectrum of applications
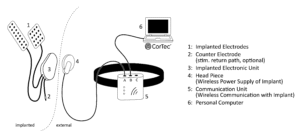
While stroke rehabilitation is the initial focus, CorTec’s technology holds promise in a wide range of indications, including epilepsy, spinal cord injuries, ALS, and treatment-resistant depression. Future applications could extend to Parkinson’s disease, pain management, and psychiatric disorders. Early academic collaborations are already underway. Alongside its BCI programme, CorTec also runs a component-based business, developing electrodes and implantable technologies for external partners in the medical device industry. This business segment is currently growing by around 40% annually and helps support the company’s capital-intensive neurotechnology development.
Made in Germany –internationally validated
The first implantation took place in the US, primarily due to funding programmes: the study is supported by the National Institutes of Health (NIH). Nevertheless, the ‘Made in Germany’ label remains central to CorTec’s identity; the company is the first to bring a German-developed BCI system into clinical evaluation. ‘We provide the first fully adaptive, semi-invasive BCI system with real-time intelligence, now in clinical validation and unique in its closed-loop functionality. This is German engineering at its finest,’ Desiere summarises.
Looking ahead to a new funding round in 2026
Reaching market approval will still take time: the current study involves twelve patients, followed by larger-scale pivotal trials. Yet CorTec is already garnering considerable interest from physicians and researchers worldwide. To finance these next steps, the company is preparing a new funding round for early next year. Its long-term ambition is to establish the technology not just as a niche solution, but as a platform and a standard for a wide spectrum of neurological disorders – ultimately providing millions of patients worldwide with access to groundbreaking therapies.






